
Precision Agriculture and Hyperspectral Sensors: Monitoring Against Drought, Disease, and Nutrient Stress
- On July 9, 2024
Crop monitoring for nutrients, water-stress, disease, insect attack and overall plant health is a vital aspect of successful agricultural operations. Traditionally this has been carried out by visual examination of crops on the ground or sometimes from the air. However these methods are limited by the ability of the human eye to discriminate between healthy foliage and foliage suffering various kinds of stress. Often a specific condition must be well-advanced before visual symptoms become noticeable even to experienced observers.
Modern precision agriculture relies on site-specific management tactics to maximize yield and resources while reducing environmental impacts such as over-fertilization and the broad applications of pesticides. Pin-pointing areas requiring attention – be it water, weed or pathogen treatment, or nutrient adjustments – allows for spot application rather than whole-field treatment. The collection of key data at a sufficient level of accuracy depends on the availability of equipment that can be operated at a cost-effective level.
Some of the benefits of hyperspectral and multispectral imaging are that these technologies are: low cost (when compared with traditional scouting methods), give consistent results, simple to use, allow for rapid assessments, non-destructive, highly accurate, and have a broad range of applications. The development of aerial and ground-based hyperspectral and multispectral imaging equipment has been a major breakthrough in the expansion and practical application of precision agriculture techniques. This technology has made possible the assessment of crop stresses, characterization of soils and vegetative cover and yield estimation, in addition to its predictive capabilities. Some of the benefits of hyperspectral and multispectral imaging are that these technologies are: low cost (when compared with traditional scouting methods), give consistent results, simple to use, allow for rapid assessments, non-destructive, highly accurate, and have a broad range of applications.[1]
Basics of Hyperspectral Imaging
Spectral reflectance, measured by hyperspectral imaging equipment, is the amount of reflected light from a surface. Hyperspectral imaging is the process by which images are taken and numerical values (spectral radiance) assigned to each pixel, utilizing a range of wavelengths across the electromagnetic spectrum, including visible and infrared regions.
Through the use of specialized software and statistical analysis, these pixels are sorted and characterized to distinguish between groups of pixels or in the case of precision agriculture, plant characteristics and environmental conditions. Earlier remote sensing technology, in particular multispectral imaging, collects data at a few widely-spaced wavelengths.The data from each wave-length band is assembled into a three-dimensional hyperspectral ‘data cube’ for processing and analysis. Each layer of the cube represents data at a specific wavelength.
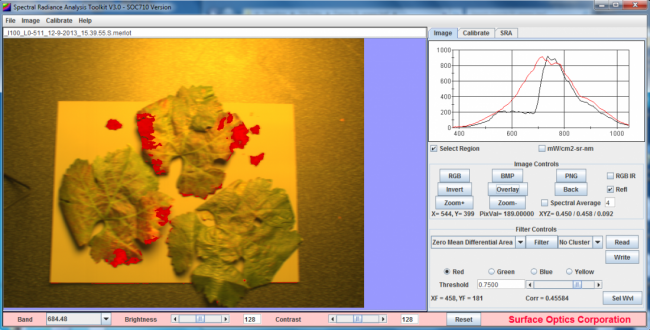
Detection of Stress-related Spectral Variations
The ability of hyperspectral imaging to provide valuable data on the condition and health of crops is predicated on the interaction and relationship between electromagnetic radiation (EMR) and foliage. EMR may be absorbed, transmitted or reflected and although the internal and external physical structure of vegetation affects this, the primary influences on EMR are the various photosynthetic pigments.[2]
In the red and blue parts of the visible spectrum, reflectance is primarily a result of absorption by the photosynthetic pigments. Water content is the primary influence on reflectance in the mid-infrared (MIR) while reflectance in the near-infrared area (NIR) is influenced by the shape and condition of air spaces in the spongy mesophyll.[3] Senescence, nutrient stress, pathogen and insect infestation have all been shown to significantly reduce reflectance in the mid-infrared spectral region.[6] It has been well recorded that a vegetation index of NIR and red wavelengths can monitor a range of plant-health issues including fungal pathogens, excess salt and nutrient deficiencies.
One of the most powerful techniques for the measurement of overall photosynthetic efficiency and thus of plant productivity, is the fluorescence of chlorophyll a in photosystem II. The indexes produced give a good measure, however they are limited in their use by the need for the active excitation of photosynthesis by, for example, a saturating light pulse.[4] This severely restricts the possibility of using this measure for remote sensing, so research has been directed to finding new, genuinely remote indices suitable for hyperspectral imaging equipment.
Besides the photosynthetic pigments, reflectance is also influenced by the presence of zeaxanthin. This pigment is produced by plants to safely remove excess photons when light intensity exceeds the ability of photosystem II to absorb photons without becoming over-energized. Zeaxanthin accumulation can therefore be used as a quantitative indicator of non-photochemical energy dissipation and therefore of light-use efficiency.[4]
By measuring changes at waveband 531nm, which is affected by the production of zeaxanthin and comparing it with waveband 570 nm, which is not affected, a standard Photochemical Reflectance Index (PRI) has been developed which serves as a measure of photosynthetic light use efficiency. This index can be readily generated from hyperspectral imaging data.[5]
Of particular importance is a comparison with traditional spectrometric equipment to ensure the ability of hyperspectral imaging to deliver equivalent data to traditional equipment. A study by Rascher et al. (2007) utilizing the SOC-700 instrument showed that portable hyperspectral imaging equipment could be used to “quantify dynamic, biochemical changes in photosynthetic efficiency” by measuring PRI.[5]
In this study it was demonstrated that PRI could provide measurements of both the biochemical adaptions to high light intensity and the gradual de-activation of photosynthesis during drying, making PRI monitoring by remote sensing a valuable methodology for drought investigations. The Rascher study relied on detached leaves, but the same methodology and instrumentation has been shown in the controlled conditions of Biosphere 2 to give effective data on whole vegetation-canopies.[4]
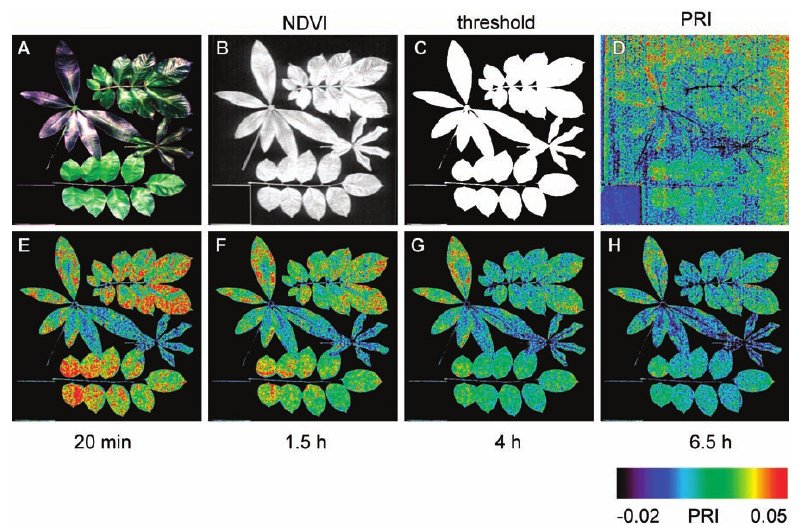
Drought Stress
Drought is a significant factor in predicting crop yields and the final success of a crop. Early detection of water related stresses in field crops can allow producers to identify specific areas for irrigation, saving water, energy, and time. Early detection might also allow producers to deliver water to crops before drought stress results in yield losses.
Colombo et al. (2008) tested various indices of drought stress and found that leaf reflectance in the infrared and visible spectrum was related to changes in leaf equivalent water thickness (EWT). Using hyperspectral imaging they tested various models for estimating EWT at the canopy level in Italian poplar plantations and found suitable models giving error levels of only 2.6%. They concluded that hyperspectral regression indices derived from hyperspectral imaging were strong tools for estimating water content at both leaf and landscape level.[7]
Higher levels of stress do ultimately manifest themselves in changes in photosynthetic pigments. These changes lead to the familiar symptom of chlorosis when the reflectance of red wavelengths increases to equal that of green, producing the typical yellow colour. These changes are detected much earlier by hyperspectral imaging well before any change is visible to the human eye.[2]
As discussed above, Rascher et al. used the Photochemical Reflectance Index and a portable hyperspectral imager to assess drought stress in leaves of tropical trees and they could clearly observe the effect of dehydration over time on the individual tree leaves.[5]
That this stress-detection methodology could be applied to grain was demonstrated in trials of corn subjected to different water and nutrient regimes in field plots. Though the traits of leaf and canopy water stress were subtle, hyperspectral imaging technology could distinguish between treatments in both the controlled and field experiments.[8] Even with senescing leaves of barley due to flowering, the SOC-700 hyperspectral imager was able to track the development of water stress four days before the effects of the stress were observed with the naked eye. Under the field conditions, with variability of light and plot differences, the imaging technology could correctly characterize three out of the four treatment groups.This demonstrated the suitability of hyperspectral imaging for early detection of drought-stress and nutrient-stress in practical agricultural conditions.
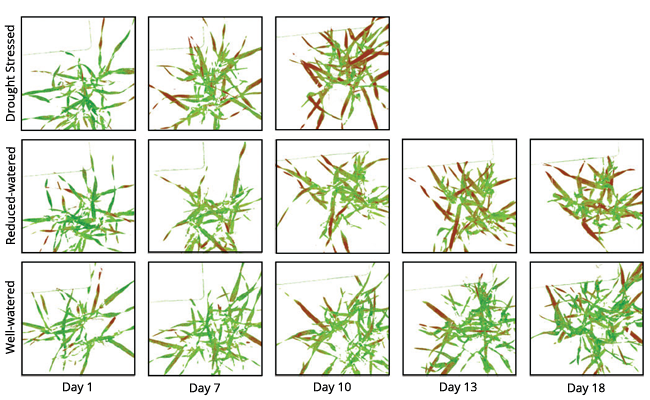
Additionally, Rossini et al. (2013) showed that hyperspectral imaging could be used to detect drought stress at the farm level with corn. They conducted a comparison of three irrigation conditions with airborne remote sensing equipment and found that they were able to accurately map irrigation deficits even before water stress affected the canopy structure.[9]
Field crops are not the only application of hyperspectral imaging, this technology can be used to assess water stress and scheduling of irrigation in turf grasses. Jiang and Carrow (2005) examined the correlation of spectral reflectance and drought stress on turf grass (turf quality and leaf firing). They screened 12 grasses and found that the reflectance models varied by cultivar, suggesting that species differences should be taken into account when using indexes. They also conclude that hyperspectral imaging might be useful in screening grasses for drought tolerance.[10]
Plant Pathogens
Fungal pathogens cause serious losses to yields and quality of agricultural crops globally. In the United States alone, plant pathogens are reported to cause economic losses of 33 billion annually.[11] Early detection of plant disease in the field can allow producers to rapidly treat affected areas and to more accurately predict yield losses. Conventional methods of detection rely on scouting and visual examination and often result in detection after the optimum time for control has passed.
In addition to preventing individual producer losses, early detection will allow for the prevention of spread to neighboring fields or crops. Using diagnostic symptoms of pathogens such as changes in leaf pigments, leaf structure and moisture content, hyperspectral and multispectral imaging can aid in mapping fields for plant disease management.
For example, the fungal pathogen, Puccinia recondite, causes wheat rust characterized by small brown pustules on the leaf surface. In a greenhouse study, wheat plants were inoculated and then hyperspectral and multispectral imaging technologies were compared for their accuracy in distinguishing treated plants from non-treated plants. Franke et al. (2005) reported that both hyperspectral and multispectral imaging proved efficient at detecting leaf rust as few as five days post inoculation.
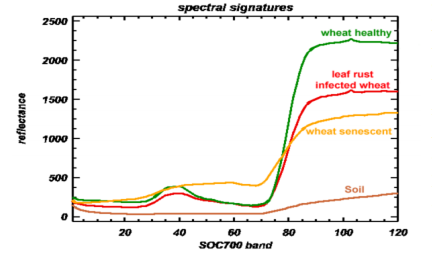
A comparison of relative reflectance between healthy and leaf rust infected wheat spectral signatures from 400-1000 nm showed a decreased reflectance for the infected wheat in the blue and green region of the visible spectrum and a strong decreased near-infrared reflectance plateau.
Compared to multispectral imaging, the higher spectral sensitivity of hyperspectral imaging produced superior detection at an earlier stage of development of the pathogen, when only slight visual symptoms were apparent. In addition, hyperspectral imaging was much less sensitive to external factors such as illumination conditions than multispectral imaging was. This prevented the poor classification accuracy found in the multispectral imaging data sets.[12]
Venturia inaequalis is the pathogen responsible for apple scab in apple trees. Infection first appears as yellow or chlorotic spots on leaves progressing to darker spots and yellowing of the leaves. Economic losses are caused primarily by damage to the fruit surfaces. Using hyperspectral imaging and statistical procedures for classification, Delalieux et al (2007) concluded that stress from apple scab was able to be detected before symptoms were visible to the human eye.[13]
Under controlled conditions, Mahlein et al (2012) reported that hyperspectral imaging was suitable for, not only the detection, but also the identification and quantification of fungal diseases of sugar beets at the leaf level. This study examined three pathogens of sugar beets, Cercospora leaf spot (Cercospora beticola (Sacc.)), powdery mildew (Erysiphe betae (Vanha) Weltzien), and sugar beet rust (Uromyces betae (Persoon) Lev.). Hyperspectral imaging technology was able to distinguish between these three pathogens with as little as 10% diseased leaf area for powdery mildew and leaf spot.[14]
The use of hyperspectral imaging can be applied successfully on a larger scale. Orange rust of sugarcane (caused by Puccinia kuehnii) is a fungal disease that produces lesions which rupture allowing water to escape from the plant. From images taken at the field level, Apan et al. (2004) successfully discriminated patches of orange rust of sugarcane from non-affected areas by detecting changes in leaf pigments.[15] Similarly, Zhang et al. (2003) demonstrated the effectiveness of hyperspectral imaging for disease detection at the field level. Late blight of tomato, caused by Phytophthora infestans, is a major threat to tomato production in California. Using bands in the range of 0.7–0.9mm, these authors demonstrated that late blight could be successfully detected in tomatoes at the field level.[16]
In a 2005 study, the SOC-700 was able to detect wheat leaf rust days before it was visible with the naked eye. Early detection allows for increased efficiency in applying site-specific fungicide application.
Ecological Monitoring
Following on from the verification of the Photochemical Reflectance Index (PRI) for whole canopies discussed above, studies have addressed the deficiencies of current ecological monitoring systems to measure global carbon utilization.[4] Although current models can accurately obtain data on overall light intensity in the appropriate range (400-700 nm) as well as the fraction of light being absorbed, they cannot obtain accurate data on light-use efficiency. Without this third data-set, models of global carbon contain substantial uncertainties which restrict their usefulness. The development of PRI, in combination with hyperspectral imaging, provides a new methodology to obtain more accurate data on light-use efficiency of whole-canopies on a global scale.
This methodology offers promise for monitoring large areas of agricultural land for photosynthetic efficiency, which translates into land output and yield data of great value for food production estimations.
Nutrient Stress
Nutrient stress in plants causes various symptoms that may be measured by the use of hyperspectral or multispectral imaging. Both deficiencies in nutrients and heavy metal contamination of soils can be assessed with this technology. Schuerger et al (2002) measured zinc deficiency and toxicity in Bahia grass by using a hyperspectral imager to determine plant chlorophyll levels correlated with stress symptoms.[17] They state that the use of this technology could map a contaminated site for a much lower cost than traditional direct sampling methods. Similarly, mercury levels in mustard plants was assessed by Dunagan et al (2006) and spectral reflectance values were significantly correlated with levels of the contaminant.[18]
In addition to investigating contamination, another application of hyperspectral imaging is to determine areas in a crop field that are nutrient poor so that fertilizer inputs could be minimized and directly targeted to nutrient poor areas. Nitrogen and phosphorus are the major yield limiting nutrients in midwestern U.S. field crops (non-leguminous). Osborne et al. (2002) describes how hyperspectral imaging can be used to estimate nitrogen and phosphorous concentrations, biomass and yield under these nutrient stresses. One important finding of this study was that timing of the images was critical to making accurate estimations of yield.[19]
Analysis of Soil Properties
The analysis and mapping of soil characteristics is also possible with hyperspectral and multispectral imaging. Maps of soil properties can improve precision agriculture technologies and enhance capabilities. Researchers in Israel were able to determine soil properties, even for soils under vegetation, with the use of hyperspectral sensors. Ben-Dor et al (2000) mapped soil organic matter, moisture, and soil salinity in a field scale experiment.[20] Rossel and Bratney (2008) accurately predicted soil organic carbon in a case study in Australia[21], and soil salinity was mapped in several locations in Europe by Farifteh et al. (2007)[22], both using hyperspectral imaging techniques.
Conclusion
The possibilities for these types of studies related to precision agriculture are virtually endless as indexes for each species, nutrient or soil property continue to be developed and improved. Studies have been conducted to estimate yield in corn by taking images during the midgrain filling stage and developing yield maps.[23] And, Okamoto and Lee (2009) demonstrated that immature fruits in orchards of oranges could be detected on individual trees.[24] Assessment of chilling, heat or insect injuries in the field would be another use of this technology in yield estimation. Currently, many other applications of hyperspectral and multispectral imaging are being tested in: post-harvest quality control, grading, and sorting of agricultural products, insect and contaminant detection, and numerous other uses in food safety. Hyperspectral imaging delivered by lower-cost, portable devices that still deliver high-quality accurate data has become a vital tool for researchers and farmers. The ability of these devices to enhance and enable day-to-day monitoring promises to create a new paradigm of agricultural efficiency.
1. Chen, Y. R., Chao, K., & Kim, M. S. (2002). Machine vision technology for agricultural applications. Computers and electronics in Agriculture, 36(2), 173-191. https://afrsweb.usda.gov/SP2UserFiles/Place/12657500/2002-Publications/2002Chen36-173-191CompElecInAgric.pdf
2. Little, Christopher and Summy, Kenneth. (2012). Accurate Spectral Measurements and Colour Infrared Imagery of Excised Leaves Exhibiting Gaussian Curvature from Healthy and Stressed Plants. [book auth.] Dr. Dimitiri Ventzas (Ed.). Advanced Image Acquisition Processing Techniques and Applications I. Rijeka : InTech, 7.
3. Lillesand, T.M., Kiefer, R.W. and Chipman, J. (2007). Remote sensing and image interpretation. New York : John Wiley and Sons, Inc. 0-47005-245-7.
4. Rascher, Ewe, Nichol, Caroline J and Small, Christopher. (2005). Hyperspectral Imaging Of Photosynthesis From the Single Leaf to the Complex Canopy – Understanding The Spatio-Temporal Variations Of Photosynthesis Within a Drought-Stressed Tropical Canopy. [ed.] B Zagajewski, M Sobcsak and Wrzesien. Warsaw : EARSel Warsaw University. Proceedings of 4th EARSeL. Workshop on Imaging Spectroscopy. pp. 709-719.
5. Rascher, Ewe, et al. (2007). Monitoring Spatio-temporal Dynamics of Photosynthesis With a Portable Hyperspectral Imaging System. Photogrammetric Engineering & Remote Sensing, Vol. 73, 1, pp. 45-56.
6. Wiegand, C.L., Smith, D.B, and Harlan, J.C. (1972). Physiological factors and optical parameters as bases of vegetation discrimination and stress analysis. Houston, Texas : American Society of Photogrammetry, Falls Church, Virginia, USA. Proceedings of the seminar on: Operational Remote Sensing.
7. Colombo, R, et al. (2008). Estimation of leaf and canopy water content in poplar plantations by means of hyperspectral indices and inverse modeling. Remote sensing of environment, Vol. 112, 4, pp. 1820-1834.
8. Römer, Christoph, et al. (2012). Early drought stress detection in cereals: simplex volume maximisation for hyperspectral image analysis. Functional Plant Biology, Vol. 39, 11, pp. 878-890.
9. Rossini, M., Fava, F., Cogliati, S., et al. (2013). Assessing canopy PRI from airborne imagery to map water stress in maize. ISPRS journal of photogrammetry and remote sensing, 86, 168-177.
10. Jiang, Y., & Carrow, R. N. (2005). Assessment of narrow-band canopy spectral reflectance and turfgrass performance under drought stress. HortScience, 40(1), 242-245. http://hortsci.ashspublications.org/content/40/1/242.full.pdf
11. Sankaran, S., Mishra, A., Ehsani, R., & Davis, C. (2010). A review of advanced techniques for detecting plant diseases. Computers and Electronics in Agriculture, 72(1), 1-13. http://128.227.177.113/pa/Publications/Sankaran_Mishra_Ehsani_Davis_2010.pdf
12. Franke, Jonas, et al., et al. (2005). Comparison of multi- and hyperspectral imaging data of leaf rust infected wheat plants. Remote Sensing for Agriculture, Ecosystems, and Hydrology VII, 5976. doi:10.1117/12.626531.
13. Delalieux, S., Van Aardt, J. A. N., Keulemans, W., Schrevens, E., & Coppin, P. (2007). Detection of biotic stress (< i> Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. European Journal of Agronomy, 27(1), 130-143. http://www.cis.rit.edu/DocumentLibrary/admin/uploads/CIS000017.pdf
14. Mahlein, A. K., Steiner, U., Hillnhütter, C., Dehne, H. W., & Oerke, E. C. (2012). Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant methods, 8(1), 3. http://www.plantmethods.com/content/8/1/3/
15. Apan, A., Held, A., Phinn, S., & Markley, J. (2004). Detecting sugarcane ‘orange rust’ disease using EO-1 Hyperion hyperspectral imagery. International journal of remote sensing, 25(2), 489-498. http://eprints.usq.edu.au/2899/1/Apan_etal_2004_IJRS_hyperion_sugarcane.pdf
16. Zhang, M., Qin, Z., Liu, X., & Ustin, S. L. (2003). Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. International Journal of Applied Earth Observation and Geoinformation, 4(4), 295-310. http://essi.nju.edu.cn/zhihao_qin_NJU/files/JAG_tomato_stress.pdf
17. Schuerger, A. C., Capelle, G. A., Di Benedetto, J. A., Mao, C., Thai, C. N., Evans, M. D., Richards, J.T., Blank, T.A. & Stryjewski, E. C. (2003). Comparison of two hyperspectral imaging and two laser-induced fluorescence instruments for the detection of zinc stress and chlorophyll concentration in bahia grass (Paspalum notatum Flugge.). Remote sensing of environment, 84(4), 572-588. http://plantpath.ifas.ufl.edu/faculty/statewide/schuerger/Schuerger_2003_RSE_Bahia_Zn.pdf
18. Dunagan, S. C., Gilmore, M. S., & Varekamp, J. C. (2007). Effects of mercury on visible/near-infrared reflectance spectra of mustard spinach plants (Brassica rapa P.). Environmental Pollution, 148(1), 301-311.
19. Osborne, S. L., Schepers, J. S., Francis, D. D., & Schlemmer, M. R. (2002). Detection of phosphorus and nitrogen deficiencies in corn using spectral radiance measurements. Agronomy Journal, 94(6), 1215-1221.
20. Ben-Dor, E., Patkin, K., Banin, A., & Karnieli, A. (2002). Mapping of several soil properties using DAIS-7915 hyperspectral scanner data-a case study over clayey soils in Israel. International Journal of Remote Sensing, 23(6), 1043-1062. http://www.bgu.ac.il/bidr/research/phys/remote/Papers/2002-Eyal_DAIS_IJRS_02.pdf
21. Gomez, C., Viscarra Rossel, R. A., & McBratney, A. B. (2008). Soil organic carbon prediction by hyperspectral remote sensing and field vis-NIR spectroscopy: An Australian case study. Geoderma, 146(3), 403-411.
22. Farifteh, J., Van der Meer, F., Atzberger, C., & Carranza, E. J. M. (2007). Quantitative analysis of salt-affected soil reflectance spectra: A comparison of two adaptive methods (PLSR and ANN). Remote Sensing of Environment,110(1), 59-78. http://www.rali.boku.ac.at/fileadmin/data/H03000/H85000/H85700/publications/27_Farifteh_et_al_2007_RSE.pdf
23. Shanahan, J. F., Schepers, J. S., Francis, D. D., Varvel, G. E., Wilhelm, W. W., Tringe, J. M., … & Major, D. J. (2001). Use of remote-sensing imagery to estimate corn grain yield. Agronomy Journal, 93(3), 583-589.
24. Okamoto, H., & Lee, W. S. (2009). Green citrus detection using hyperspectral imaging. Computers and Electronics in Agriculture, 66(2), 201-208. http://sensinglab.engr.uga.edu/twiki/pub/SensingLab/AcademicDevelopment/HiroshiOkamoto_2009_GreenCitrusHSI.pdf



0 Comments